Introduction
Prostate volume constitutes a fundamental parameter in urological practice, influencing diagnostic pathways, risk stratification and therapeutic decision-making. Accurate assessment of gland size underpins evaluation of benign prostatic hyperplasia (BPH) progression, guides prostate-specific antigen (PSA) density calculations and informs surgical planning. This article examines the clinical significance of prostate volume through historical context, current measurement modalities, applications in BPH and prostate cancer management, emerging technologies and limitations, concluding with key considerations for future practice.
Historical Perspectives on Prostate Volume Assessment
Early quantification of prostatic enlargement relied on digital rectal examination (DRE) and empirical estimation. The advent of transrectal ultrasonography (TRUS) in the 1980s introduced objective imaging-based metrics. Subsequent refinements included magnetic resonance imaging (MRI)-based segmentation, which enabled zonal volume analysis. As B. Garvey et al. observed, “The progression of BPH can be quantified by measuring the volumes of the whole prostate and its zones, based on image segmentation on magnetic resonance imaging.” [Garvey et al.]
Techniques for Prostate Volume Measurement
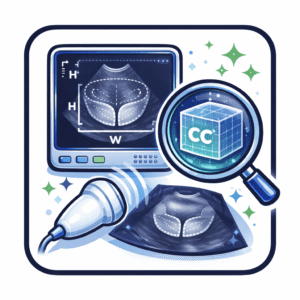
Contemporary practice employs three principal methods for prostate volume measurement:
- Prolate ellipsoid formula applied to TRUS or MRI dimensions
- Bullet formula, a modified ellipsoid approach
- Planimetry, involving manual or automated contour tracing
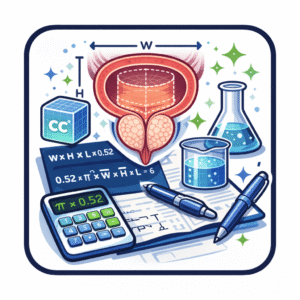
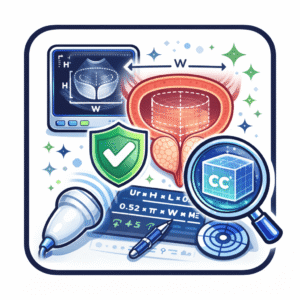
The standard ellipsoid approach defines volume (V) in cubic centimeters (cc) as the product of three orthogonal diameters—anteroposterior (AP), transverse (TR) and craniocaudal (CC)—multiplied by 0.523:
Volume (cc) = AP × TR × CC × 0.523 [J Urol. 1992;148(6):1736–40]
This formula exhibits “excellent correlation with pathologic specimens (r = 0.89) despite systematic underestimation by approximately 17 percent,” indicating robustness in routine clinical use. MRI-based segmentation methods offer improved accuracy—interclass correlation coefficient (ICC) 0.91 versus 0.71 for TRUS—but are constrained by cost, availability and scanning time. Automated deep-learning frameworks for transabdominal ultrasound (TAUS) are under investigation; early results suggest potential for non-invasive, rapid estimation without catheterization or sedation. [Natali et al.]
Prostate Volume in Benign Prostatic Hyperplasia
BPH represents a non-malignant proliferation of prostatic glandular and stromal elements, predominantly within the transitional zone. Prevalence approaches 50 percent by age 60 and escalates with advancing age. Volume expansion correlates imperfectly with symptom severity but correlates strongly with progression risk. S. Ahmad et al. reported that, for clinically relevant volumes (> 30 cc), “94.5 percent patients were accurately estimated on DRE,” indicating that even low-resource settings can stratify patients effectively. Their cohort further established three volume categories for therapeutic guidance:
- Prostate volume < 30 cc: candidates for α-blocker monotherapy
- Prostate volume > 30 cc: candidates for combination therapy with 5α-reductase inhibitors (5-ARIs)
- Prostate volume > 80 cc: surgical approaches such as holmium laser enucleation
DRE demonstrated a “positive predictive value of 94 percent in identifying prostate above 30 cc,” providing a rapid triage tool when imaging is unavailable. [Ahmad et al.]
Prostate Volume and Prostate-Specific Antigen Density
PSA density (PSAD) normalizes serum PSA to gland size, mitigating confounding by benign enlargement. PSAD is computed as:
PSAD (ng/ml/cc) = Serum PSA (ng/ml) ÷ Prostate Volume (cc) [Cancer. 2004;100(4):683–5]
In diagnostic performance comparisons, PSAD consistently outperforms absolute PSA:
- Clinically significant prostate cancer detection: PSAD AUC 0.78 vs. PSA AUC 0.64 (p < 0.001)
- PSA gray zone (4–10 ng/ml): PSAD sensitivity 65 percent, specificity 85.2 percent at threshold 0.155 ng/ml/cc
- Elevated PSA (> 10 ng/ml): PSAD sensitivity 96.6 percent, specificity 66.7 percent at threshold 0.175 ng/ml/cc
- Negative MRI with elevated PSA: PSAD AUC 0.848 vs. PSA AUC 0.722 (p = 0.04)
Role in Prostate Cancer Risk Stratification
Total gland size influences both biopsy decision-making and active surveillance eligibility. Multiple guidelines incorporate PSAD cut-offs (e.g., < 0.15 ng/ml/cc for surveillance candidacy). In PI-RADS 3 lesions on multiparametric MRI, PSAD refines risk, with higher densities indicating greater likelihood of clinically significant carcinoma. Large volumes may dilute PSA, obscuring early neoplasia; hence, precise volume assessment is critical in men with discrepant PSA and imaging findings. [PI-RADS v2.1 update]
Planning of Therapeutic Interventions
Volume thresholds inform selection among medical, minimally invasive and surgical options:
- 5-ARI therapy demonstrates maximal efficacy in glands > 40 cc, achieving volume reduction and symptom relief (MTOPS study)
- Combination α-blocker and 5-ARI therapy shows superior outcomes in prostates > 30 cc (CombAT trial)
- Brachytherapy seed loading and dosimetry require pre-implant volume measurement
- Holmium Laser Enucleation (HoLEP) and open prostatectomy are considered when volume exceeds 80 cc
Emerging Imaging Modalities and Automated Estimation
Advances in artificial intelligence have yielded frameworks for automatic prostate volume estimation from TAUS images, aiming to combine non-invasiveness with operator independence. Tiziano Natali et al. described a “deep-learning-based framework for automatic PV estimation using TAUS,” highlighting potential to democratize accessible volume metrics in primary care settings. [Natali et al.]
Limitations and Areas for Improvement
Despite technological progress, measurement variability persists:
- TRUS-based ellipsoid assumptions introduce systematic underestimation (~ 17 percent) relative to pathology.
- DRE estimates lack precision in intermediate volumes (25–30 cc and > 80 cc), necessitating imaging cross-verification.
- Inter-observer variability in planar measurements remains significant; standardized training and protocol adherence are essential.
- Access to MRI segmentation tools and deep-learning platforms is limited by cost and technical expertise.
Final Considerations
Precise assessment of prostate volume underpins management across the spectrum of prostatic disease. The calculation of prostate volume via standardized formulas or segmentation techniques enhances diagnostic accuracy, risk stratification and tailored intervention planning. As imaging modalities evolve, integration of automated volume estimation holds promise for broader access and consistency. However, awareness of methodological limitations and adherence to validated protocols remain crucial for reliable data generation. Clinicians should interpret volume metrics within the broader clinical context, combining imaging, PSA kinetics and patient factors to optimize outcomes.