Why Measurement Method Shapes Clinical Reality
Prostate volume appears in medical records as a single figure, usually expressed in milliliters or cubic centimeters. That apparent simplicity masks a complex chain of assumptions, approximations, and technical decisions. The method used to measure prostate volume influences diagnostic thresholds, treatment selection, procedural planning, and longitudinal follow-up. Volume is not an inherent property waiting to be read; it is an estimate produced through technique.
Understanding how prostate volume is measured, and where each method performs well or poorly, forms a necessary foundation for interpreting clinical data responsibly. This examination focuses on the dominant measurement methods in current practice, their empirical performance, and the implications of relying on one method rather than another.
Anatomical and Geometric Constraints
The prostate lacks regular geometry. It shows asymmetric growth, lobular enlargement, and deformation related to benign hyperplasia, inflammation, or malignancy. Measurement methods rarely capture this complexity directly. Most rely on geometric approximation.
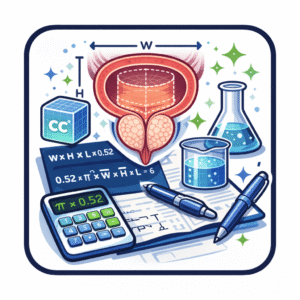
The prevailing mathematical approach models the gland as an ellipsoid using the formula:
Volume = length × width × height × π / 6
This formula remains embedded in ultrasound machines, radiology software, and nearly every prostate volume calculator in routine use. Its endurance reflects practicality rather than anatomical precision. The accuracy of any effort to calculate prostate volume depends less on the formula itself than on how dimensions are obtained.
Digital Rectal Examination: Historical Context
Before imaging became routine, clinicians estimated prostate size through digital rectal examination. This method persists as a screening tool, not a quantitative measurement strategy.
Studies comparing digital estimates with imaging demonstrate substantial error. Research published in The Journal of Urology showed that digital rectal examination underestimated prostate volume in larger glands by more than 30% in many cases.
https://www.auajournals.org
Digital examination informs clinical suspicion, not numerical assessment. Its role in volume measurement has become largely historical.
Transrectal Ultrasound (TRUS)
Technique and Workflow
Transrectal ultrasound places a high-frequency probe into the rectum, producing axial and sagittal images of the prostate. Measurements of length, width, and height are taken manually, then processed through an ellipsoid formula to calculate prostate volume.
TRUS gained dominance through accessibility, speed, and cost efficiency. It remains standard in biopsy settings and outpatient urology.
Accuracy and Limitations
Empirical evaluation of TRUS reveals consistent patterns:
- Underestimation in prostates above 50 mL
- Overestimation in smaller glands
- Operator-dependent variability exceeding 15%
Probe pressure alters anterior-posterior dimensions, and slice selection affects maximal width. A 2015 study in Urology found discrepancies exceeding 20% between TRUS-derived volumes and prostatectomy specimens in nearly one-third of cases.
https://www.goldjournal.net
TRUS-based measurement represents convenience rather than precision. A prostate volume calculator using TRUS inputs reproduces these limitations faithfully.
Magnetic Resonance Imaging (MRI)
Methodological Advantages
Multiparametric MRI offers superior soft-tissue contrast, enabling clearer delineation of the prostatic capsule, transition zone, and peripheral zone. MRI volume estimation relies on either ellipsoid approximation or planimetric segmentation across sequential slices.
Planimetry involves outlining the prostate on each slice, summing cross-sectional areas multiplied by slice thickness. This approach reduces geometric assumptions.
Performance Data
Multiple studies demonstrate improved accuracy with MRI. A systematic review in European Urology reported mean absolute errors below 10% when MRI-derived volumes were compared with surgical specimens.
https://www.europeanurology.com
Reproducibility improves when experienced radiologists perform manual segmentation. Automated segmentation tools show promise yet remain sensitive to protocol variation.
The European Association of Urology states that MRI provides the most reproducible prostate volume measurements available for PSA density assessment.
https://uroweb.org/guidelines/prostate-cancer
Computed Tomography (CT)
CT imaging captures prostate anatomy incidentally rather than intentionally. Soft-tissue contrast limits boundary definition, particularly at the apex and base.
Comparative studies show CT systematically overestimates prostate volume relative to MRI and surgical specimens. Errors arise from partial volume effects and indistinct margins.
CT holds limited value for dedicated prostate volume measurement and remains absent from guideline recommendations.
Transabdominal Ultrasound
Transabdominal ultrasound estimates prostate size via a suprapubic approach. Bladder filling influences image quality and gland visualization.
Accuracy suffers in obese patients and in those with large prostates. Comparative research indicates wider error margins than TRUS, with limited reproducibility. Its role remains restricted to preliminary assessment in primary care settings.
Surgical Specimen Measurement: Reference Standard
Radical prostatectomy specimens allow direct measurement using water displacement or pathological sectioning. These methods provide the closest approximation to true prostate volume.
Surgical measurements expose systematic biases in imaging-based methods. A landmark analysis in The Journal of Urology demonstrated that ellipsoid-based imaging estimates underestimated true volume by 7–12% on average.
https://www.auajournals.org
Surgical volume measurement functions as a benchmark rather than a clinical tool, limited to retrospective validation.
Planimetric Versus Ellipsoid Techniques
Ellipsoid Approximation
Ellipsoid methods persist across imaging modalities. Their advantages include speed and simplicity. Their disadvantages emerge in irregular glands.
Errors increase with:
- Median lobe enlargement
- Asymmetric hyperplasia
- Post-inflammatory distortion
Planimetric Segmentation
Planimetric methods reduce shape assumptions. They demand time, expertise, and high-quality imaging.
Studies comparing ellipsoid and planimetric MRI techniques show planimetry improves accuracy by 5–8 percentage points.
https://pubmed.ncbi.nlm.nih.gov
Clinical adoption remains uneven, constrained by workflow considerations.
Automated and AI-Driven Measurement
Machine learning algorithms increasingly assist prostate segmentation. Early validation studies report Dice similarity coefficients exceeding 0.90 when compared with expert manual contours.
Performance declines in:
- Very large prostates
- Glands altered by surgery or radiation
- Imaging protocols outside training datasets
The National Cancer Institute emphasizes validation across populations before clinical reliance.
https://www.cancer.gov
Automated tools support consistency rather than replace expert oversight.
Measurement Method and PSA Density
PSA density divides serum PSA by prostate volume. Volume measurement error propagates directly into PSA density.
A 10% underestimation of volume inflates PSA density equivalently, shifting patients across biopsy thresholds. Guideline thresholds referenced by the American Urological Association depend on accurate volume input.
https://www.auanet.org/guidelines
Method selection shapes downstream risk stratification.
Longitudinal Measurement Consistency
Serial measurements provide insight into growth velocity and treatment response. Consistency in modality matters more than absolute accuracy across modalities.
Switching from TRUS to MRI introduces artificial changes unrelated to biological growth. Studies show apparent volume increases of 10–20% attributable solely to modality change.
Longitudinal interpretation benefits from method stability.
Practical Comparison of Methods
Key attributes across measurement methods include:
- Digital rectal examination: qualitative only
- Transrectal ultrasound: accessible, operator dependent
- MRI: highest reproducibility, higher cost
- CT: limited accuracy
- Transabdominal ultrasound: preliminary assessment
- Surgical specimen: reference standard
Selecting a method involves balancing precision, availability, and clinical purpose.
Implications for Clinical Decision-Making
Measurement method influences:
- Eligibility for 5-alpha-reductase inhibitor therapy
- Selection of minimally invasive procedures
- PSA density interpretation
- Active surveillance thresholds
Errors propagate silently through decision pathways. A prostate volume calculator streamlines computation, not measurement quality.
Final Considerations
Prostate volume measurement rests on technique rather than certainty. Each method carries predictable biases shaped by anatomy, physics, and workflow. Precision improves through advanced imaging, disciplined segmentation, and consistency over time.
Efforts to calculate prostate volume achieve clinical value only when their methodological limits remain visible. Numbers guide decisions, yet understanding how those numbers arise defines responsible practice.