Why Prostate Volume Measurement Carries Clinical Weight
Prostate volume measurement occupies a central role in modern urology. It informs diagnostic thresholds, medication choices, eligibility for minimally invasive procedures, and longitudinal monitoring of benign prostatic hyperplasia and prostate cancer. A few milliliters can alter prostate-specific antigen density, shift risk stratification, or determine whether a patient meets criteria for surgical intervention.
Against this background, digital tools marketed as a prostate volume calculator promise speed and standardization. They reduce manual arithmetic and claim reproducibility. Their reliability, though, depends entirely on the assumptions, formulas, and imaging data behind them. Accuracy varies by method, modality, and operator behavior, raising a critical question: how close do calculator-derived volumes come to anatomical reality?

Mathematical Foundations Behind Volume Estimation
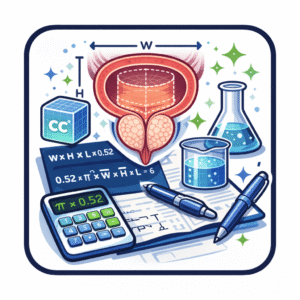
Nearly all tools designed to calculate prostate volume rely on geometric approximation rather than direct measurement. The dominant approach remains the ellipsoid model, expressed as:
Volume = length × width × height × π / 6
This formula has been embedded for decades in ultrasound machines, radiology reporting software, and online calculators. Its appeal lies in simplicity and speed. The prostate is treated as a regular ellipsoid even though anatomical studies show asymmetry, lobulation, and distortion from hyperplasia or malignancy.
A secondary approach substitutes a constant of 0.52 for π/6. The mathematical difference is negligible; practical differences arise from how the three dimensions are acquired.
Digital calculators do not improve accuracy on their own. They automate arithmetic, not anatomy. Error originates at the measurement stage, not the computation stage.
Imaging Modalities and Their Error Profiles
Transrectal Ultrasound (TRUS)
Transrectal ultrasound remains widely used in outpatient urology. It is inexpensive, fast, and familiar. Volume estimates obtained through TRUS underpin many prostate volume calculators currently in use.
Peer-reviewed studies consistently demonstrate systematic bias with TRUS:
- TRUS tends to underestimate larger prostates and overestimate smaller ones
- Inter-operator variability frequently exceeds 15%
- Probe pressure and imaging angle alter measured height and width
A 2015 study published in Urology reported that TRUS-derived volumes differed from surgical specimen volumes by more than 20% in nearly one-third of cases. The calculator used was mathematically correct; the inputs were not.
Magnetic Resonance Imaging (MRI)
Multiparametric MRI has altered prostate imaging standards. Its superior soft tissue contrast allows clearer delineation of the capsule and transition zone.
MRI-based volume estimates show improved correlation with prostatectomy specimens:
- Mean absolute error often falls below 10%
- Reduced operator dependence compared to TRUS
- Improved consistency across institutions
Radiology software that includes a prostate volume calculator benefits from this higher-quality input, though segmentation method still matters. Manual contouring produces more accurate volumes than automated boundary detection in irregular glands.
The European Association of Urology notes in its clinical guidance that MRI provides “the most reproducible prostate volume measurements currently available,” particularly when PSA density is used for risk assessment.
https://uroweb.org/guidelines/prostate-cancer
Surgical Specimens as the Reference Standard
The most accurate prostate volume measurement occurs after radical prostatectomy, when the gland can be measured directly via water displacement or pathological sectioning. These values serve as the benchmark against which calculators and imaging methods are judged.
Comparative studies show:
- Ellipsoid formulas systematically underrepresent anterior-posterior asymmetry
- Errors increase with median lobe enlargement
- Shape distortion from benign hyperplasia reduces geometric validity
A landmark analysis in The Journal of Urology found that ellipsoid-based estimates underestimated true volume by a median of 7–12%, even when MRI was used for dimension acquisition.
https://www.auajournals.org
Calculator Design and Embedded Assumptions
Most online and software-based tools appear neutral, but design choices influence output:
- Fixed constants assume average gland shape
- Lack of adjustment for transition zone hypertrophy
- No correction for imaging modality differences
Some calculators silently round measurements, truncating decimals. Others default to centimeters without unit confirmation. These choices introduce compounding inaccuracies, especially when volume is used to derive secondary metrics.
PSA density illustrates the problem. PSA divided by prostate volume is highly sensitive to volume error. A 10% underestimation of volume inflates PSA density by the same margin, potentially shifting a patient across biopsy thresholds recommended by the American Urological Association.
https://www.auanet.org/guidelines
Clinical Decision-Making and Risk of Misclassification
In daily practice, prostate volume calculators influence several decisions:
- Alpha-blocker versus 5-alpha-reductase inhibitor selection
- Suitability for minimally invasive therapies
- Interpretation of PSA trends
- Eligibility for active surveillance protocols
Volume inaccuracies propagate downstream. Overestimation may delay treatment escalation; underestimation may prompt unnecessary intervention.
A multicenter cohort study published in European Urology reported that PSA density misclassification occurred in 18% of patients when TRUS-based volumes were substituted for MRI-based volumes. In nearly half of those cases, management recommendations changed.
https://www.europeanurology.com
Inter-Observer Variability: The Human Factor
No calculator corrects for inconsistent measurement technique. Studies consistently show that operator experience shapes results:
- Junior operators produce wider variance in height measurements
- Probe compression affects sagittal dimensions
- Slice selection alters maximal width
Training reduces but does not eliminate variance. Even expert radiologists show measurable disagreement when outlining gland borders manually.
The National Cancer Institute emphasizes that reproducibility, not theoretical precision, determines clinical utility. Their imaging guidance notes that consistency across serial measurements carries more weight than absolute accuracy when monitoring progression.
https://www.cancer.gov
Emerging Technologies and Algorithmic Segmentation
Artificial intelligence tools promise automated prostate segmentation. Early validation studies report encouraging performance, with Dice similarity coefficients exceeding 0.90 when compared to expert contours.
Still, algorithmic bias remains a concern:
- Training datasets underrepresent large prostates
- Performance drops in glands distorted by prior surgery
- Vendor-specific MRI protocols affect output
Until external validation matures, AI-driven calculators function as adjuncts rather than replacements for expert review.
Regulatory and Reporting Standards
No regulatory body certifies the accuracy of consumer-facing prostate volume calculators. Most disclaim medical use while being routinely used in clinical contexts.
Radiology reporting standards increasingly recommend documenting:
- Measurement method
- Imaging modality
- Calculation formula
Such transparency allows clinicians to interpret volume values within appropriate uncertainty bounds.
Practical Guidance for Clinicians and Researchers
Evidence supports several pragmatic strategies:
- Prefer MRI-based measurements when volume influences thresholds
- Maintain consistent modality across follow-up
- Avoid comparing TRUS-derived and MRI-derived volumes interchangeably
- Treat calculator output as an estimate, not a fact
Calculators remain tools, not authorities. Their accuracy reflects input quality, not interface sophistication.
Final Considerations
Prostate volume calculators occupy an ambiguous space between convenience and precision. They simplify arithmetic while masking the biological complexity of gland morphology. Their outputs influence diagnoses, treatment pathways, and patient anxiety, yet remain approximations shaped by geometry and operator judgment.
Accuracy improves with better imaging, disciplined measurement technique, and awareness of embedded assumptions. It erodes when calculators are treated as neutral arbiters rather than computational shortcuts.
Clinical judgment begins where calculator certainty ends.